STUDIES
Preclinical Data
Vepoloxamer’s multimodal effects enhance the therapeutic ratio in radiation therapy by mitigating oxidative stress, reducing inflammation and protecting healthy tissues.
Study: Velopoxamer inhibiting the onset of acute pneumonitis in a rat model of radiation-induced pneumonitis
Tested Properties:
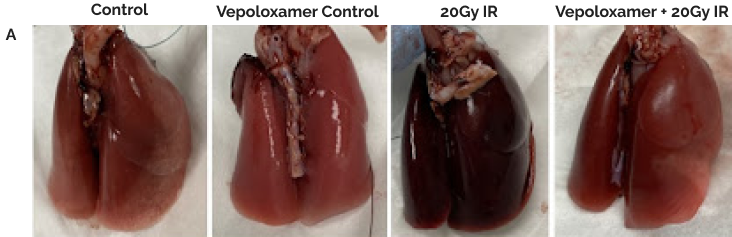
(A). Representative gross lung images for all animal groupings.
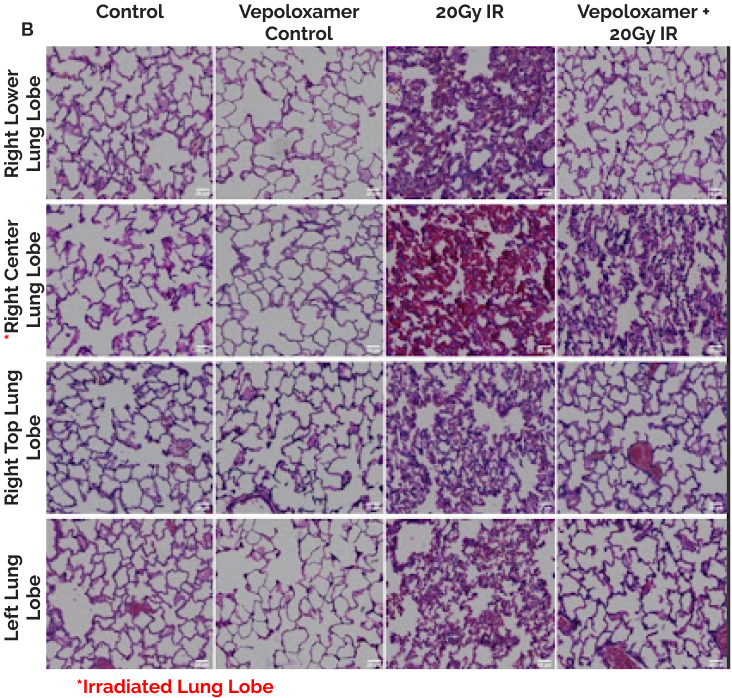
(B). Representative images of all lung lobes bilaterally stained with hematoxylin and eosin at 6-weeks post-20Gy irradiation to the right center lung lobe (6mm); scale bar 200μm. Without Vepoloxamer, animals developed the hallmark signs of acute radiation-induced pneumonitis including evident edema and significant alveolar wall thickening that was not localized to the irradiated area, but spread to all lung lobes bilaterally. With Vepoloxamer, the irradiation damage is limited to the directly exposed right center lobe*, while other lobes appear healthy and normal.
Study: Velopoxamer inhibiting radiation-induced chronic inflammation in healthy tissues
Tested Properties:
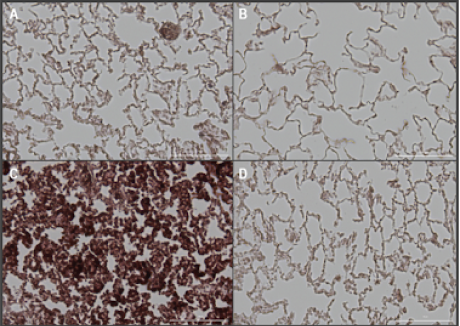
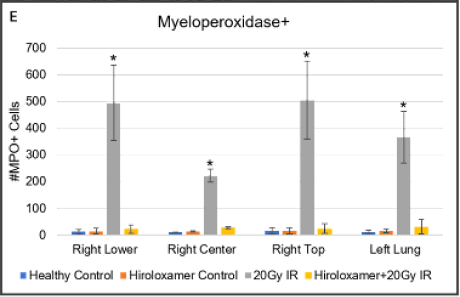
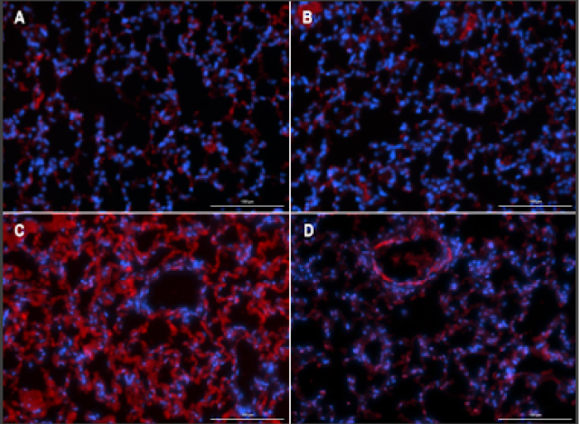
(A-D) Representative images of right lower lung lobes stained with Hanker-Yates Peroxidase Leukocyte kit at 6-weeks post-irradiation; scale bar 100μm. (A) Healthy Control, (B) Vepoloxamer control, (C) 20Gy IR, (D) Vepoloxamer + IR.
(E) Quantified levels of myeloperoxidase-positive cells in all lung lobes bilaterally using automated thresholding within the Gen5 analysis software. The data are presented as mean ± SD (n=20 per lobe). *P<0.001, using one-way ANOVA followed by Tukey’s multiple comparisons post-test.
Tissue damage was observed in the control 20Gy IR animals, that had significantly elevated levels of MPO in all lung lobes compared to the Vepoloxamer+IR and healthy normal (D).
Study: Velopoxamer mitigating damage to healthy tissue
Tested Properties:
(A) Representative images of right lower lung lobes, adjacent to irradiated center lobe. The significant inflammatory infiltrate observed in irradiated animals that did not receive Vepoloxamer contributed to pathological changes within major lung structures.
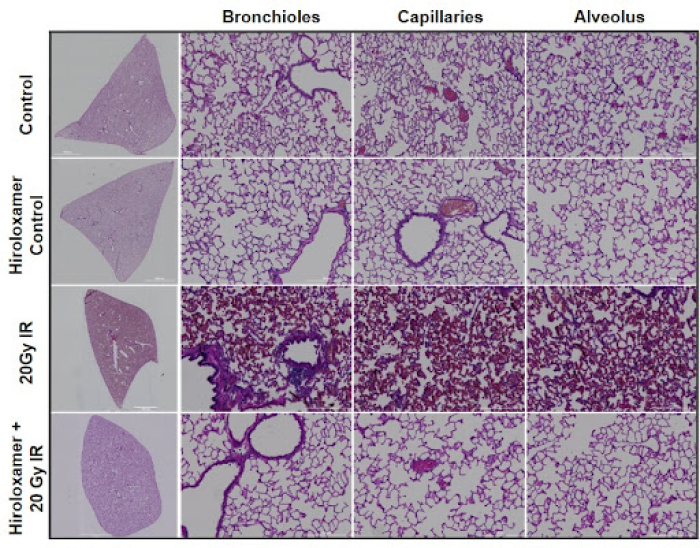
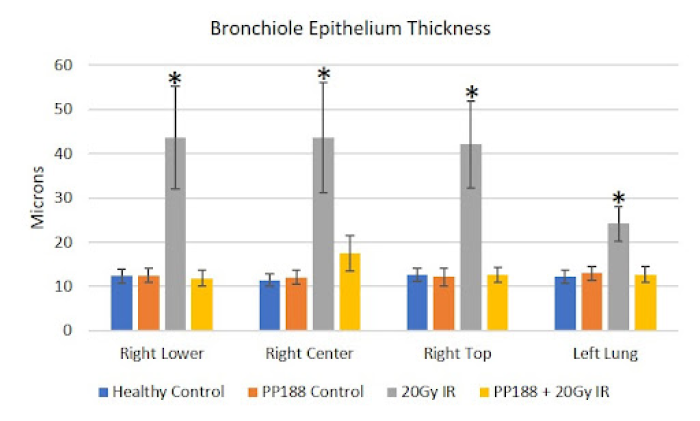
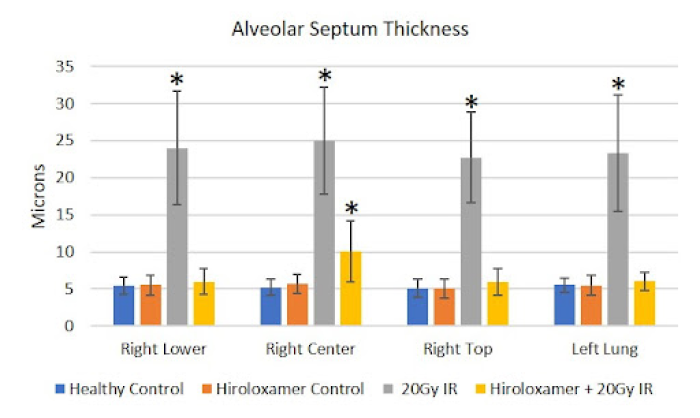
(B) Significant vascular changes were observed with significant bronchiole epithelium thickening observed in all lung lobes bilaterally (p<0.001), as well as congestion of the lung parenchyma, the alveolar septa, which constitutes 90% of the lung volume.
(C) With Vepoloxamer treatment, there was significant alveolar wall thickening within the irradiated right center lung lobe (p<0.001) that was limited to this area. The other three lung lobes, including right top, right center, and left lung, were not significantly different from controls.
Study: Velopoxamer preventing myofibroblast activation
Tested Properties:
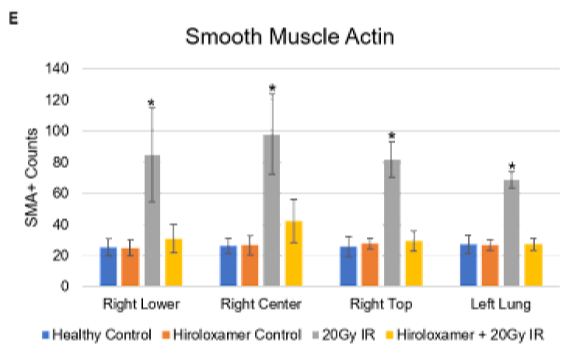
(A-D) Representative images (20X) of alpha-smooth muscle actin (red, 1:500, Sigma, A2547) and Hoechst (blue, 1:10000, ThermoFisher Scientific, 33342)-stained right lower lung lobe sections, adjacent to irradiated right center lung lobe of (A) Healthy control, (B) Vepoloxamer control, (C) 20 Gy IR, and (D) Vepoloxamer+20Gy IR; scale bar 100μm.
(E) Quantified counts of alpha-smooth muscle actin-positive cells via thresholding in ImageJ. Results are presented as mean ± SD (n=5 for each lobe). *P<0.001, using one-way ANOVA followed by Tukey’s multiple comparisons post-test.
There were low levels of SMA expression in control, Vepoloxamer control, and Vepoloxamer+IR groups due to the resident fibroblasts and myofibroblasts within the lung parenchyma (Figure 5). Without Vepoloxamer, irradiation induced SMA accumulation in the alveoli of all lung lobes bilaterally at significantly elevated levels of expression (p<0.001). Vepoloxamer treatment prevented the accumulation of α-SMA in the alveolar septa that was observed in the saline+RT group.
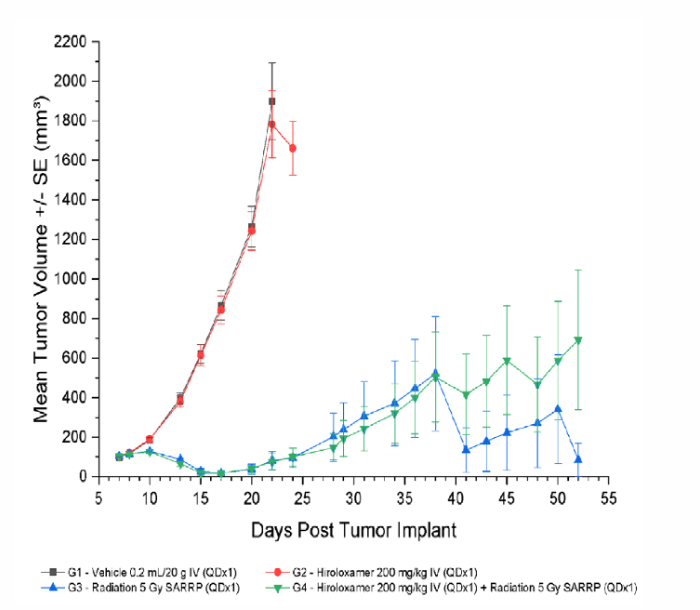
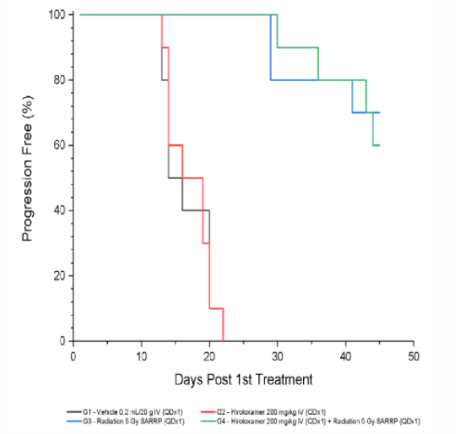
Results: Radiotherapy + Vepoloxamer Effectively Reduces Tumor Volume
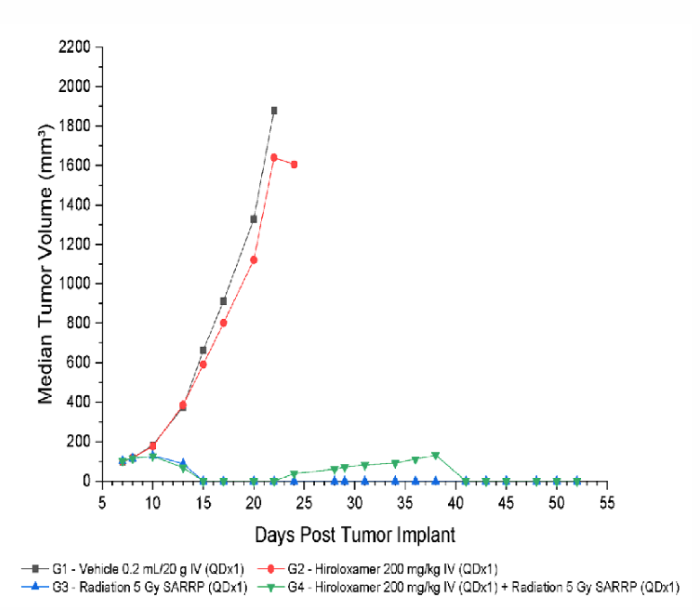
Results: Treatment with Vepoloxamer did not negatively impact radiotherapy outcomes
Treatment with Vepoloxamer alone (0 Gy; Group 2) resulted in an increase in time to progression of 13%. Treatment with focal radiation (5 Gy; Group 3) and radiation + Vepoloxamer (5 Gy + 200 mg/kg; Group 4) were efficacious resulting in an increase in time to progression of 200%, complete regression of 80%, and tumor-free survivor of 60% and 50%, respectively.
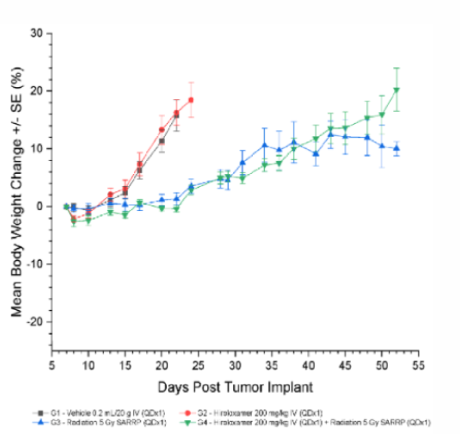
Results: Vepoloxamer Treatment is Well Tolerated
All treatments were well tolerated with minimal weight loss (between -2.1 and -2.6) in the treatment window and no incidence of treatment related deaths.